![]()
How CONQUEST helps the scleroderma community
New scientific advancements have brought to light many new understandings of the mechanisms of action—specifically, the drivers of autoimmunity and fibrosis—and have generated a rich pipeline of promising therapeutics across autoimmunity and specifically, for scleroderma. However, among other issues, the breadth and depth of opportunities poses clinical development challenges in a rare disease setting such as scleroderma.
CONQUEST arrives at the right moment to address those challenges and offers unique benefits for scleroderma patients. It also offers huge efficiencies to encourage more clinical trials and more rapid evaluation of potential therapies for scleroderma.
ENROLLMENT INFORMATION
Join the quest to advance systemic sclerosis research through CONQUEST.
Consider enrolling in the CONQUEST study, a scleroderma clinical trial for people with systemic sclerosis-associated interstitial lung disease (SSc-ILD). Participants help progress research to potentially uncover new SSc-ILD treatment options.

What are the benefits of CONQUEST to scleroderma patients?
CONQUEST is a unique opportunity for patients for the following reasons:
- Efficient trial design to study multiple therapies with a go/no-go decision
- Be part of drug discovery/biomarker validation/outcomes measures development and validation
- 2:1 randomization active to placebo. Increased chance of randomization to an active arm (compared with other trial designs)
- Availability of placebo data to the broad scleroderma medical science community (to improve design and timelines iteratively)
Who is eligible to participate in the clinical trial?
As in all clinical trials, the CONQUEST protocol identifies what are called entry criteria, detailing the profiles of patients needed to evaluate the potential new drugs. Patients can learn more about CONQUEST by checking back here.
The SRF supports this global effort. We are using our global social media reach and news outlets to create awareness in the scleroderma community worldwide.
Where is CONQUEST happening?
A few trial sites in the United States have already begun recruitment. More than 150 sites worldwide in over 30+ countries will participate in CONQUEST. Additional trial centers will be based in Europe, North America, Asia, and South America.
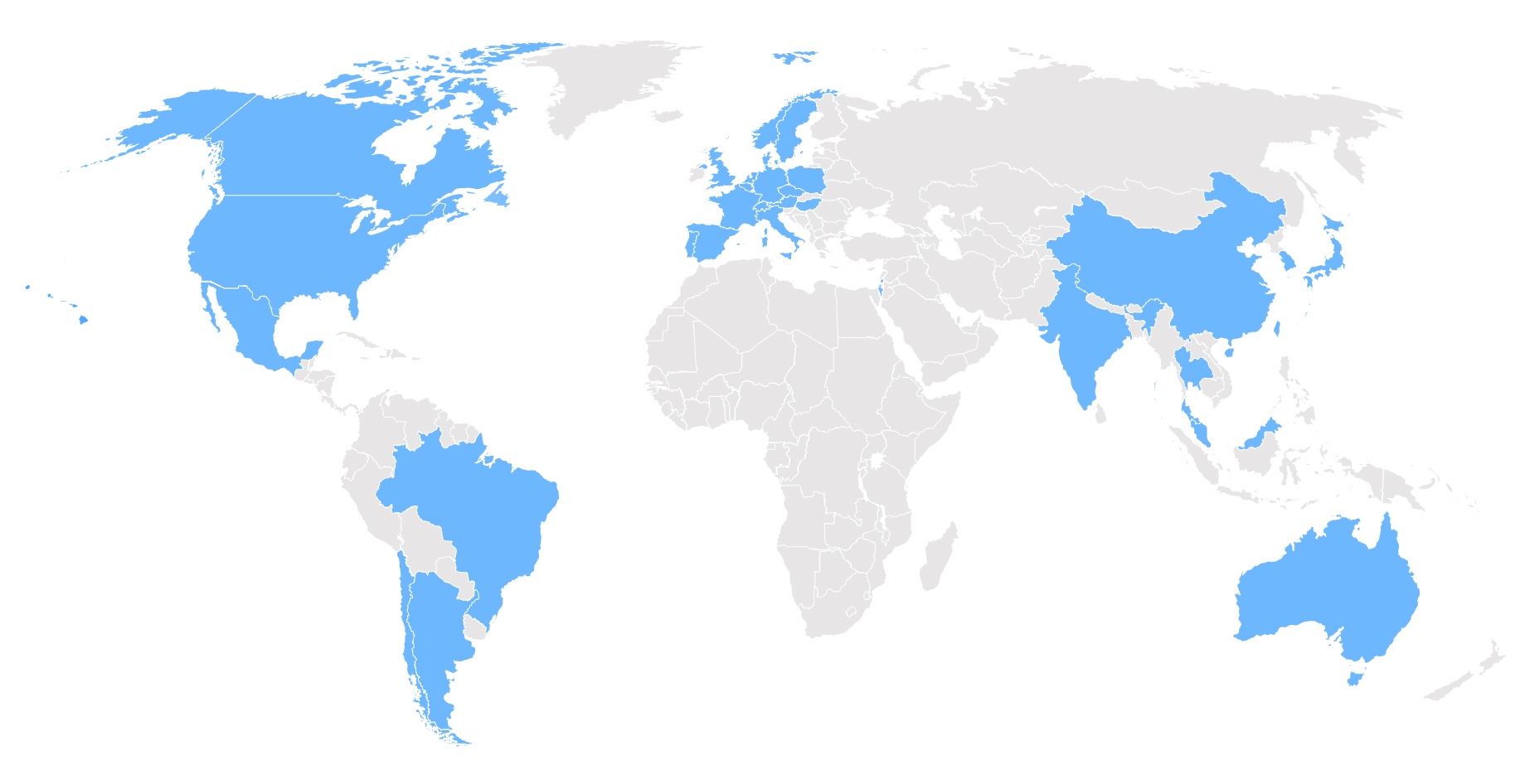
Anticipated Countries
Argentina
Australia
Austria
Belgium
Canada
Chile
China
Denmark
France
Germany
India
Israel
Italy
Japan
Malaysia
Mexico
Netherlands
Norway
Poland
Portugal
South Korea
Spain
Switzerland
Taiwan
Thailand
United Kingdom
United States
5+ Additional Countries
Learn more about CONQUEST’s impact on patients
From the 2023 SRF Patient Forum
More about CONQUEST.
About Conquest
CONQUEST is designed to rapidly advance promising treatments for scleroderma.
The CONQUEST Steering Committee
Meet the people providing external oversight of CONQUEST.
CONQUEST FAQ
Read our answers to our most frequently asked questions about CONQUEST.
LIVING WITH SCLERODERMA
View resources for people living with scleroderma.
Whether you have been newly diagnosed or have lived with scleroderma for many years, we hope to help you learn about scleroderma, understand how it is diagnosed, and find ways to treat it.
Contact
If you are a healthcare provider or pharmaceutical company, please email info@conquestssc.org
If you are a person with scleroderma or a caregiver, please email info@sclerodermaresearch.org


