![]()

What Is The CONQUER Registry?
In 2018, The SRF launched the CONQUER Registry (an acronym for COllaborative National QUality and Efficacy Registry) – a first-of-its-kind nationwide longitudinal scleroderma patient registry and biosample repository aimed at improving care and developing more effective, personalized therapies for systemic sclerosis (scleroderma) patients. Scleroderma can manifest in many different ways.
Why is the CONQUER Registry needed?
Some patients have fibrotic skin disease while others do not. Some patients have lung disease, heart disease, or gastrointestinal disease while other patients have many affected organs. Why is this and why is it so difficult for physicians to predict an individual patient’s disease course? Similarly, scleroderma patients need and are treated with many different therapies that span from simply monitoring the disease coupled with symptomatic therapy (e.g. H2 blockers and PPIs for heartburn/reflux) all the way to aggressive therapy aimed at modifying their underlying disease, such as chemotherapeutic immune system resetting with autologous stem cell rescue.
What is the right therapy for any one scleroderma patient?
What is the range of outcomes that patient might expect?
Unfortunately, the answers to critical questions such as these are not fully understood and comprehensive data does not yet exist to allow researchers to evaluate them.
The path to answering these questions requires tracking and collecting data on the health status, disease complications, treatments, and outcomes of many patients over many years. The Scleroderma Research Foundation began developing the CONQUER Registry in 2013 with the goal of defining the epidemiology of scleroderma in the current era by enrolling a large group of patients and building a powerful database to track them. It is now the first nationwide longitudinal scleroderma patient registry in the U.S. Data will be collected over the course of the normal clinic visits – ultimately for thousands of patients over many years. The data generated through the CONQUER Registry will allow researchers to refine disease subsets (going beyond “diffuse” and “limited”) and track patient outcomes for each subset with the aim of enabling more precise and tailored care for individual patients.
About the Program
Due to the rarity of scleroderma, a broad consortium of scleroderma centers is required to enroll a sufficiently large patient population and assemble a sufficiently comprehensive data set to enable deeper insights into the disease. International scleroderma groups have launched large observational studies in Europe, Canada, and Australia; however, these do not necessarily reflect the U.S. scleroderma patient population and, in any case, have some methodological flaws that compromise their utility.
In 2011, the Prospective Registry of Early Systemic Sclerosis (PRESS) registry was developed in the U.S. in response to the FDA’s and the NIH’s calls for attention to Therapeutics for Rare and Neglected Diseases and developing tissue banks linked to clinical outcomes. The PRESS investigators were approached by the Scleroderma Research Foundation (“SRF”) to build upon their efforts. The overarching goal of the partnership of the SRF and the participating academic Scleroderma Centers is to amass the broadest possible cohort of early-stage scleroderma patients. This highly collaborative effort, initially among 12 (now 19) of the largest scleroderma centers in the U.S., has been named CONQUER (an acronym for COllaborative, National QUality and Efficacy Registry for Tracking Disease Progression in Systemic Sclerosis (scleroderma) Patients).
The CONQUER Registry became operational in 2019. As of June 2024, more than 1,000 patients have enrolled in CONQUER, contributing invaluable data to the search for a cure.
Leadership
It takes a community of dedicated scientists and clinicians to create and carry out a project of this size and scale. The SRF is grateful to the following participating institutions and principal investigators who lend their expertise and leadership to make the CONQUER Registry possible.
| Institution | Principal Investigator |
|---|---|
| Boston University Medical Center | Andrea Bujor, MD, PHD |
| Columbia University | Elana Bernstein, MD, MSc |
| Duke University | Ankoor Shah, MD |
| Georgetown University | Virginia Steen, MD |
| Hospital for Special Surgery | Jessica Gordon, MD |
| Johns Hopkins University | Ami Shah, MD Laura Hummers, MD |
| Mass General Hospital | Flavia Castelino, MD |
| Mayo Clinic | Ashima Makol, MD |
| Medical University of South Carolina | Faye Hant, DO, MSCR |
| Northwestern University | Carrie L. Richardson, MD |
| Stanford University | Lori Chung, MD, MS |
| The University of Texas Health Science Center at Houston | Shervin Assassi, MD |
| University of Minnesota | Jerry Molitor, MD |
| University of California, Los Angeles | Elizabeth Volkmann, MD, MS |
| University of Colorado, Anshutz Medical | Melissa Griffith, MD |
| University of Michigan | Dinesh Khanna, MD |
| University of Pennsylvania | Nora Sandorfi, MD |
| University of Utah | Dorota Lebiedz-Odrobina, MD |
| Vanderbilt University Medical Center | Tracy Frech, MD |
Impact
The CONQUER Registry will enable researchers to:
- Determine whether certain disease features are associated with or predict clinical and patient-reported outcomes (short-term and long-term).
- Identify which patients require early and aggressive intervention and which patients are better suited for “watchful waiting
- Evaluate the response in the real world (as opposed to the controlled and constrained setting of a clinical trial) to therapeutic agents and to combinations of therapeutic agents.
- Drive more personalized and effective therapy for patients
- Develop insights into drug toxicities that are unique to scleroderma patients (e.g. underlying heart disease due to pulmonary vascular disease)
- Better understand patient satisfaction/issues with the current Standard of Care
- Collect biosamples for future analyses (e.g. genetic factors contributing to disease)
- Establish and support a collaborative network for U.S. scleroderma investigators
- Support the critical infrastructure for future scleroderma studies, including trials for novel therapeutics
Researchers have already began publishing based on insights gleaned from CONQUER.
Collaborative National Quality and Efficacy Registry (CONQUER) for Scleroderma: Outcomes from a Multicenter US-based Systemic Sclerosis Registry (2020)
By: Victoria K. Shanmugam, Tracy M. Frech, Virginia D. Steen, Laura K. Hummers, Ami A. Shah, Elana J. Bernstein, Dinesh Khanna, Jessica K. Gordon, Flavia V. Castelino, Lorinda Chung, Faye N. Hant, Emily Startup, John M. VanBuren, Luke B. Evnin & Shervin Assassi
Does hand involvement in Systemic Sclerosis limit PRO completion? (2021)
By: Tracy M. Frech, John M. VanBuren, Emily Startup, Shervin Assassi, Elana J. Bernstein, Flavia V. Castelino, Lorinda Chung, Chase Correia, Jessica K. Gordon, Faye N. Hant, Laura Hummers, Dinesh Khanna, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia Steen & Luke Evnin
Summary: The objective of this analysis is to examine whether the severity of systemic sclerosis (SSc)-hand involvement influences patient-reported outcome measure (PROM) completion rate. There were 339 CONQUER participants with total of 600 baseline and follow-up visits available for analysis. Fingertip resorption (acro-osteolysis) and calcium deposits (calcinosis) were associated with lower PROM completion, independent of the length of the questionnaires or the modality of administration (electronic or paper). Multiple strategies are needed to ensure optimal completion of PROM in longitudinal cohort studies.
Baseline Characteristics of Systemic Sclerosis Patients with Restrictive Lung Disease in a Multi-Center US Based Longitudinal Registry (2022)
By: Flavia V. Castelino, John M. VanBuren, Emily Startup, Shervin Assassi, Elana J. Bernstein, Lorinda Chung, Chase Correia, Luke B. Evnin, Tracy M. Frech, Jessica K. Gordon, Faye N. Hant, Laura K. Hummers, Dinesh Khanna, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia Steen
Summary: The researchers looked at the various characteristics associated with restrictive lung disease (RLD) in patients enrolled in CONQUER. There were no differences in age, gender or disease duration. Patients with restrictive lung disease had a mean disease duration of 2.6 years. Non-white race, higher physician global health assessment scores and dyspnea scores were independently associated with RLD. Interestingly, 45% of CONQUER participants already had RLD, highlighting the importance of screening for lung disease at initial diagnosis of systemic sclerosis.
Computed Tomography of the Chest to Screen for Interstitial Lung Disease in Patients with Systemic Sclerosis at Expert Scleroderma Centers in the U.S. (2022)
By: Elana J. Bernstein, Shervin Assassi, Flavia V. Castelino, Lorinda Chung, Chase Correia, Luke B. Evnin, Tracy M. Frech, Jessica K. Gordon, Brian A. Skaug, Faye N. Hant, Laura K. Hummers, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia D. Steen, Dinesh Khanna
Summary: Although a chest CT scan is the gold standard test for the diagnosis of interstitial lung disease, there is no consensus among rheumatologists regarding the use of chest CT to screen for interstitial lung disease in their patients with systemic sclerosis. The goal of this study was to describe the chest CT ordering practices at expert scleroderma centers in the United States and to determine which patient characteristics are associated with obtaining a chest CT. The researchers found that 80% of systemic sclerosis patients enrolled in CONQUER underwent a chest CT. Patients with a positive centromere antibody were less likely to have undergone a chest CT.
CONQUER Scleroderma: Association of Gastrointestinal Tract Symptoms in Early Disease with Resource Utilization (2023)
By: Sarah Luebker, Tracy M Frech, Shervin Assassi, Brian Skaug, Jessica K Gordon, Kimberly Lakin, Elana J Bernstein, Yiming Luo, Virginia D Steen, Ami A Shah, Laura K Hummers, Carrie Richardson, Duncan F Moore, Dinesh Khanna, Flavia V Castelino, Lorinda Chung, Puneet Kapoor, Faye N Hant, Victoria K Shanmugam, John M VanBuren, Jessica Alvey, Monica Harding, Ankoor Shah, Ashima Makol, Dorota Lebiedz-Odrobina, Julie K Thomas, Elizabeth R Volkmann, Jerry A Molitor, Nora Sandorfi
Summary: This report from the CONQUER cohort looked at how gastrointestinal tract (GIT) symptoms impacts health-related costs of care. Among the 211 CONQUER participants in this study, most (64%) had mild GIT symptoms, 26% had moderate symptoms, and 10% severe GIT symptoms at 12-months of follow-up. More upper endoscopy procedures and inpatient hospitalization occurred in the CONQUER participants with severe GIT symptoms. It is especially important to understand symptom activity prior to damage to the GIT and how diagnosis increases care costs.
The Collaborative National Quality and Efficacy Registry for Scleroderma: Association of Medication Use on Gastrointestinal Tract Symptoms in Early Disease and the Importance of Tobacco Cessation (2023)
By: S. Luebker, T.M. Frech, S. Assassi, J.K. Gordon, E.J. Bernstein, V.D. Steen,
A.A. Shah, L.K. Hummers, C. Richardson, D. Khanna, F.V. Castelino1, L. Chung, F.N. Hant, V.K. Shanmugam, J.M. VanBuren, J. Alvey, M. Harding, N. Sandorfi
Summary: This manuscript presents the gastrointestinal tract (GIT) outcomes in relation to other scleroderma manifestations and medication exposures. 415 enrolled CONQUER participants were included in this analysis. Most participants had stable mild GIT symptoms at baseline and were prescribed immunosuppression and anti-heartburn therapy. In most patients, anti-reflux medication and immunosuppression initiation preceded the baseline visit, whereas anti-fibrotic initiation occurred at or after the baseline visit. There was no clear association between immunosuppression or anti-fibrotic use and severity of GIT symptoms. However, active tobacco use was associated with worse GIT symptoms, highlighting the importance of smoking cessation counselling.
Psychometric Evaluation of the Scleroderma Skin Questionnaire: A Novel Patient-Reported Outcome for Skin Disease in Patients With Systemic Sclerosis (2025)
By: Jeong Min Yu, John M VanBuren, Angela Child, Jessica S Alvey, Lisa A Mandl, Laura C Pinheiro, Shervin Assassi, Elana J Bernstein, Flavia V Castelino, Lorinda Chung, Luke Evnin, Tracy M Frech, Faye N Hant, Laura K Hummers, Dinesh Khanna, Kimberly S Lakin, Dorota Lebiedz-Odrobina, Yiming Luo, Ashima Makol, Jerry A Molitor, Duncan F Moore, Carrie Richardson, Nora Sandorfi, Ami A Shah, Ankoor Shah, Victoria K Shanmugam, Brian Skaug, Virginia D Steen, Elizabeth R Volkmann, Jessica K Gordon
Use of Nintedanib for the Treatment of Systemic Sclerosis-associated Interstitial Lung Disease at Expert Scleroderma Centers in the United States (2025)
By: Yiming Luo, Shervin Assassi, Maureen D Mayes, Tracy M Frech, Virginia D Steen, Carrie Richardson, Lorinda Chung, Nora Sandorfi, John M VanBuren, Monica Harding, Jessica S Alvey, Brian Skaug, Zsuzsanna McMahan, Kimberly S Lakin, Carleigh Zahn, Sonali J Bracken, Dorota J Lebiedz-Odrobina, Jerry A Molitor, Luke B Evnin, Duncan F Moore, Ankoor Shah, Flavia V Castelino, Elizabeth R Volkmann, Faye N Hant, Ashima Makol, Ami A Shah, Laura K Hummers, Jessica K Gordon, Dinesh Khanna, Elana J Bernstein
Hand swelling and other non-Raynaud's symptoms as the initial presentation of systemic sclerosis: prevalence and clinical associations in two U.S. cohorts (2025)
By: Iqtidar Hanif, Shervin Assassi, Maureen D. Mayes, Zsuzsanna H. McMahan, Meng Zhang, Julio Charles, John M. VanBuren, Jessica S. Alvey, Kimia Ghaffari, Elana J. Bernstein, Flavia V. Castelino, Lorinda Chung, Luke Evnin, Tracy M. Frech, Jessica K. Gordon, Faye N. Hant, Laura K. Hummers, Dinesh Khanna, Kimberly S. Lakin, Dorota Lebiedz-Odrobina, Yiming Luo, Ashima Makol, Jerry A. Molitor, Duncan F. Moore, Carrie Richardson, Nora Sandorfi, Ami A. Shah, Ankoor Shah, Victoria K. Shanmugam, Virginia D. Steen, Elizabeth R. Volkmann, Carleigh Zahn, Brian Skaug
Summary: This study indicates that for over 30% of people with systemic sclerosis, the earliest symptoms weren’t Raynaud’s phenomenon; instead, signs like puffy fingers or hand swelling came first.
How The CONQUER Registry Works
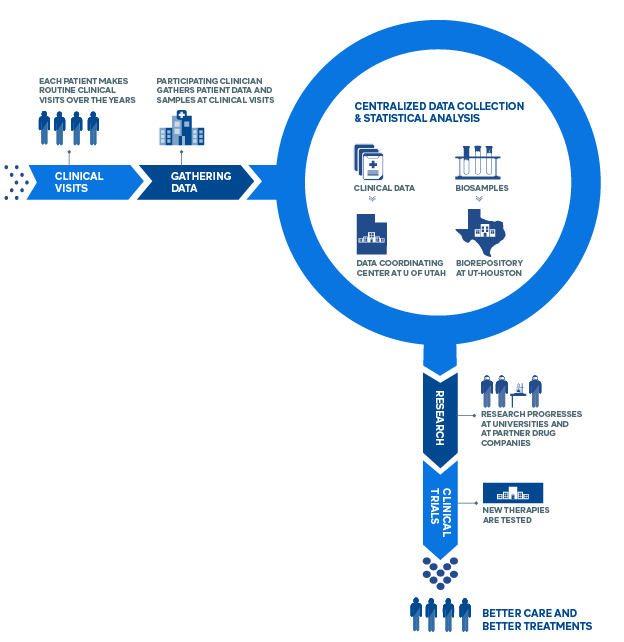
Data Coordination
The Data Coordinating Center (DCC), based at the University of Utah School of Medicine, provides a central repository for data generated by each of the CONQUER sites. The DCC also works with each of the CONQUER Principal Investigators and Research Coordinators to implement CONQUER-wide standards for data collection and analysis in order to ensure uniformity and quality of the data. The DCC implements regular monitoring activities and performs statistical analyses for many manuscripts published on behalf of CONQUER.
CONQUER Sponsors
As the founder of the CONQUER Registry, the SRF plays a lead role in the administration and financing of its activities. We are fortunate to have the support of generous corporate and community sponsors who provide financial resources and expertise to ensure the success of the CONQUER Registry. We gratefully acknowledge these corporate and community partners, and welcome new partners to join us:
Founder

Founding Sponsor

Supporting Partners



OUR RESEARCH
People with Scleroderma are Needed for Our Research
Your experience with scleroderma is invaluable. The more we can understand how this disease affects different people in different ways the closer we will get to finding a cure.

