![]()
CONQUEST: A Clinical Study From the Scleroderma Research Foundation
When working on a rare disease like scleroderma, it can be difficult for scientists and drug companies to develop strong pipelines for new treatments. The Scleroderma Research Foundation (SRF) realized this was a critical issue for the scleroderma community, and so we created CONQUEST.
CONQUEST is a platform clinical trial, which allows it to evaluate multiple investigational study drugs at the same time. It embodies a patient-first philosophy that aims to advance and accelerate clinical research and support for the scleroderma community.
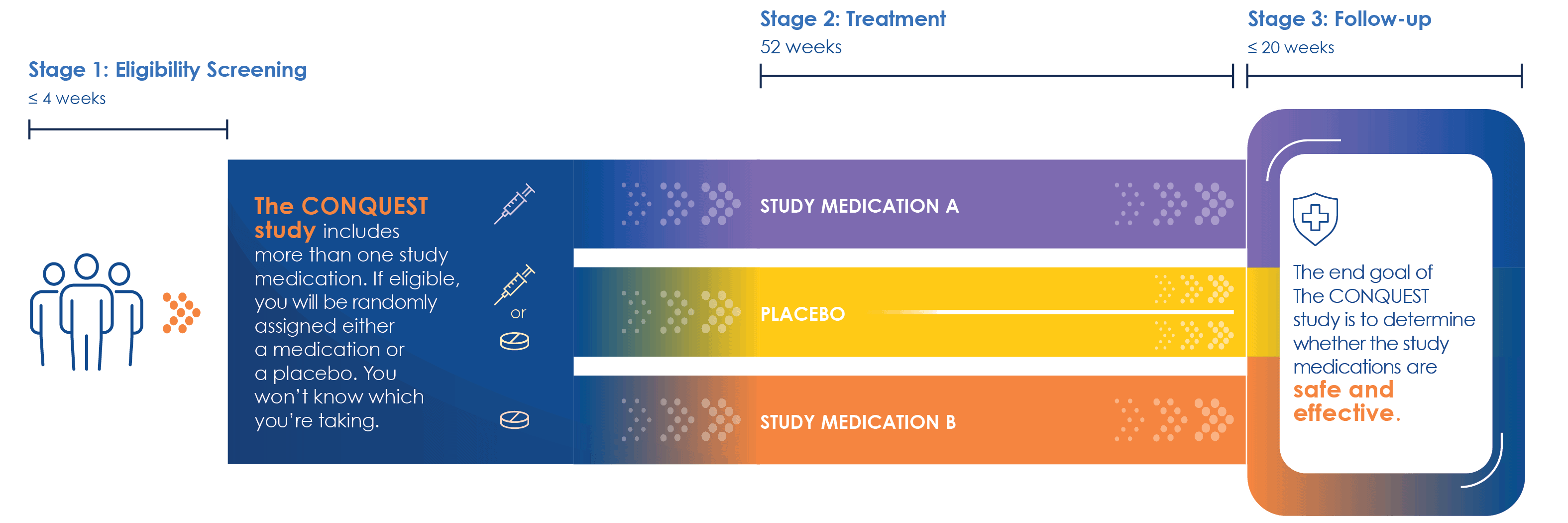
Currently, CONQUEST is evaluating two investigational study drugs for interstitial lung disease associated with systemic sclerosis (SSc-ILD). Over time, CONQUEST plans to look at more investigational study drugs for other aspects of systemic sclerosis. The end goal is to determine whether the study medications are safe and effective.
You Can Join the Quest to Advance Clinical Research
Consider enrolling in CONQUEST, a scleroderma clinical trial for people with SSc-ILD. Participants help move research forward to potentially uncover new SSc-ILD treatment options.
CONQUEST Site Map
![]() Active Site
Active Site ![]() Pending Site
Pending Site
Click on the pins for more information.
Who Is Eligible?
Right now, CONQUEST is evaluating study drugs for SSc-ILD. Here are a few key criteria participants must have for potential enrollment:
- Adults aged 18 years or older.
- SSc-ILD diagnosis.
- SSc symptoms that began within the past 7 years. (Note that it is allowed for a participant to have experienced Raynaud’s phenomenon for longer than 7 years.)
- Other criteria apply (study staff will provide details).
- Please note that the following treatments are not allowed during CONQUEST. So, if you are currently taking one of them, you will not be eligible to participate:
- Cyclophosphamide
- Tocilizumab
- Nintedanib
- Pirfenidone
- Abatacept
In the future, CONQUEST may evaluate study drugs with other focuses. That means that these criteria may change. So if you don’t currently qualify for CONQUEST, you could still potentially qualify for CONQUEST in the future.
What Can Study Participants Expect?
CONQUEST has a randomized design. If eligible to participate, patients will be randomly assigned to one of the investigational study drugs or the placebo treatment. Placebos resemble the investigational study drug but do not have the therapeutic effects. Study staff will provide additional details on the investigational study drugs and design.
Your treatment might include:
- At least 10 doctor visits
- Procedures, potentially including pulmonary function tests, HRCT (high-resolution computed tomography) scans—also known as CAT scans—and blood draws
Why Participate?
Here a just a few reasons why you might consider participating in CONQUEST:
- Contribute to Clinical Research: By enrolling in CONQUEST, you are not only helping to advance clinical research, you’re also providing hope to individuals living with scleroderma.
- Increased Chance of Study Medication: CONQUEST’s design increases participants’ chances of receiving an active study drug rather than a placebo.
- Access to Multiple Sites Worldwide: With over 150 centers across more than 25 countries participating so far, there is a good chance that there will be a study center near you. Travel support is available as needed.
What’s the Long-Term Goal?
The end goal of CONQUEST is to determine whether the study medications are safe and effective. CONQUEST is a perpetual trial, meaning it will continue to run over the coming years.
Right now, CONQUEST is focused on SSc-ILD, but in the future, it will focus on multiple, different aspects of scleroderma. The SRF aims to make a long-lasting contribution to the scleroderma community through testing more new potential therapies in CONQUEST.
Watch Now: All About CONQUEST
From the 2023 SRF Patient Forum
Frequently Asked Questions
How can I look into participating in CONQUEST?
Clinical trials have specific criteria to determine whether a person qualifies for participation. It is important to speak with your healthcare provider to discuss whether you potentially qualify for CONQUEST and whether it’s a good fit for you.
You can also take an online pre-screener survey. Click on “See if I May Qualify” to take the pre-screener and evaluate CONQUEST as an option for you.
Do I have to pay to participate in CONQUEST?
There are no costs for participating in the CONQUEST study. The Scleroderma Research Foundation will offer travel support and reimbursement through Medpace Patient Concierge Services (PCS).
Why don’t some individuals qualify for CONQUEST?
The goal with all clinical trials is to be as inclusive as possible. However, it is necessary to develop some inclusion and exclusion criteria. These allow researchers to perform a credible study to determine if a new drug can successfully treat a disease, such as scleroderma.
In most clinical trials, some participants receive active treatment, while others receive a matching placebo treatment. The inclusion and exclusion criteria are used to ensure that these two groups of participants are similar to one another. This way, any difference in disease outcome during the trial is truly due to the study treatment.
Right now, CONQUEST focuses on individuals with scleroderma who have had it for 7 years or less and who also have a diagnosis of interstitial lung disease.
Do people with other diseases in addition to scleroderma qualify for CONQUEST?
With any clinical trial, the goal is to be as inclusive as possible. For CONQUEST, any diseases a person has in addition to scleroderma will be reviewed by the investigators to determine if they impact potential eligibility for the study.
How do you measure the length of time of active scleroderma?
At this stage, individuals with a diagnosis of scleroderma with an onset of less than or equal to 7 years (≤7 years) prior to the screening visit may be candidates for CONQUEST. The length of time of active scleroderma is defined as the time from the onset of the first non-Raynaud’s symptom.
If you’re not sure how long it has been since your first symptom, the trial investigator can discuss it with your physician as part of the screening process.
What if I change my mind about participating in CONQUEST? Can I leave?
Participation in the CONQUEST study is voluntary. You can leave at any time. You always have the right to withdraw for any reason without penalty or loss of access to your regular medical care. Your safety and autonomy remain a priority throughout the study.
Contact Information
Please email info@conquestssc.org