![]()
At the Scleroderma Research Foundation, research is at the center of all we do.
What is CONQUER?
In this video, learn why scleroderma patient participation in the registry is essential for improving care and developing more effective, personalized therapies for scleroderma patients.
In 2018, The SRF launched the CONQUER Registry (an acronym for COllaborative National QUality and Efficacy Registry) – a first-of-its-kind nationwide scleroderma patient registry and biosample repository aimed at improving care and developing more effective, personalized therapies for systemic sclerosis (scleroderma) patients.
CONQUER was created through a collaborative effort among the largest scleroderma research centers in the U.S. With the goal of enrolling thousands of scleroderma patients from around the country, researchers and clinicians will be able to learn as much as possible about all forms of scleroderma in patients of all backgrounds and ethnicities and use that knowledge to advance scleroderma research and discovery.
As of June 2024, more than 1,000 patients have enrolled in CONQUER, contributing invaluable data to the search for a cure for scleroderma.
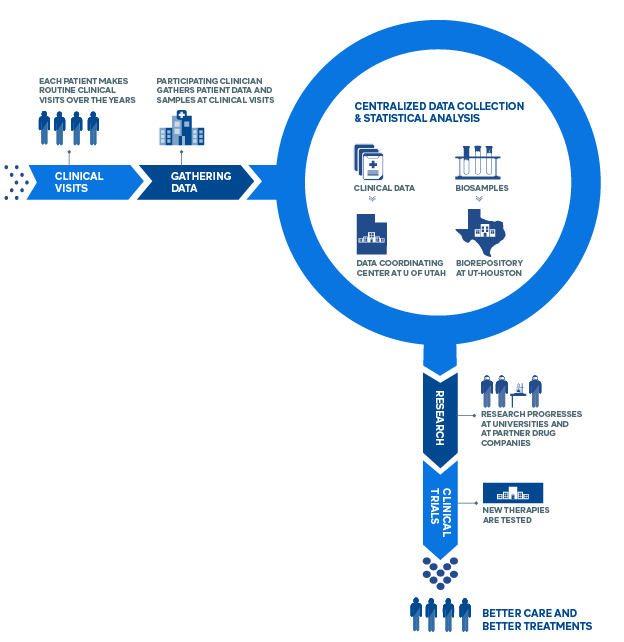
How the CONQUER Registry Works
- Scleroderma patients participate through their routine clinical visits (2x/year), contributing Clinical data & Blood samples
- Scleroderma patients also contribute data through Patient-Reported Outcome surveys. (This can be done at the patient’s convenience.)
- Enrolled scleroderma patients receive the most modern standard of care. There are no experimental therapies involved in CONQUER.
Who is Eligible to Participate?
To be eligible for CONQUER, the national scleroderma patient registry, you must:
- Be 18 years of age or older
- Have systemic scleroderma
- Be within first 5 years of first non-Raynaud’s symptom (Enrollment is time sensitive)
You can participate in CONQUER even if you are enrolled in another registry.
CONQUER will benefit every scleroderma patient, even those unable to enroll.
How Do I Participate in CONQUER?
- The 19 institutions listed below are the sites participating in CONQUER.
- We do anticipate that other expert sites will be added over time and are in the process of evaluating such additions now. If you are a patient at one of these participating centers, ask your doctor about enrolling in CONQUER.
- Otherwise, please contact the Scleroderma Research Foundation at info@srfcure.org or call us at 800.441.CURE (2873).
CONQUER Sites
Boston University Medical Center
Principal Investigator: Andrea Bujor, MD, PhD
Primary Contact Person: Rutvi Patel, Research Coordinator
rutvip@bu.edu
Columbia University, New York
Principal Investigator: Elana Bernstein, MD, MSc
Co-Investigator: Yiming Luo, MD
Primary Contact People:
Niki Pradhan, Research Coordinator
np2749@cumc.columbia.edu
Megan Santilli, Research Coordinator
ms6696@cumc.columbia.edu
212.342.6815
Georgetown University, Washington, D. C.
Principal Investigator: Virginia Steen, MD
Primary Contact Person: Destiny Barksdale, Research Coordinator
db1619@georgetown.edu
Hospital for Special Surgery, New York
Principal Investigator: Jessica Gordon, MD
Co-Investigators: Kim Lakin, MD, MS; Robert Spiera, MD
Primary Contact Person: Mia Diaz, Research Assistant
diazmia@hss.edu
212.774.7620
Duke University, North Carolina
Principal Investigator: Ankoor Shah, MD
Primary Contact People:
Karissa Grier, Research Coordinator
karissa.grier@duke.edu
Edna Scarlett, Research Coordinator
edna.scarlett@duke.edu
Johns Hopkins University, Maryland
Principal Investigators: Ami Shah, MD and Laura Hummers, MD
Primary Contact People:
Adrianne Woods, Research Coordinator
awoods9@jhmi.edu
Gwen Leatherman, Research Coordinator
gleathe@jhmi.edu
410.550.6820
Mass General Hospital, Massachusetts
Principal Investigator: Flavia Castelino, MD
Primary Contact Person: Ali Puri, Research Coordinator
apuri3@mgh.harvard.edu
507.923.0814
Mayo Clinic, Minnesota
Principal Investigator: Ashima Makol, MD
Primary Contact People:
Jenni Sletten, Research Coordinator
Sletten.Jennifer@mayo.edu
Jane Jaquith, Research Coordinator
jaquith.jane@mayo.edu
Patty Kennedy, Research Coordinator
Kennedy.Patricia1@mayo.edu
507.284.4797
Medical University of South Carolina
Principal Investigator: Faye Hant, DO, MSCR
Primary Contact Person: Brittany Frasier, Research Coordinator
frasibri@musc.edu
Northwestern University, Illinois
Principal Investigator: Carrie L. Richardson, MD
Co-Investigator: Duncan Moore, MD
Primary Contact People:
Kathleen Aren, Research Coordinator
kathleen.aren@northwestern.edu
Alexandra Soriano, Research Coordinator
alexandra.soriano@northwestern.edu
Mary Carns, Research Coordinator
m-carns@northwestern.edu
312.503.1120
Stanford University, California
Principal Investigator: Lori Chung, MD, MS
Primary Contact Person: Swarna Nandyala, Research Coordinator
swarnan@stanford.edu
The University of Texas Health Science Center at Houston
Principal Investigator: Shervin Assassi, MD
Primary Contact People:
Samuel “Sam” Theodore, Research Coordinator
samuel.theodore@uth.tmc.edu
Maya Washington, Research Coordinator
Maya.Washington@uth.tmc.edu
University of California, Los Angeles
Principal Investigator: Elizabeth Volkmann, MD, MS
EVolkmann@mednet.ucla.edu
Primary Contact Person: Paola Hernandez
PGHernandez@mednet.ucla.edu
University of Colorado, Anshutz Medical
Principal Investigator: Kristen Demoruelle, MD, PhD
kristen.demoruelle@cuanschutz.edu
Primary Contact Person: Kristin Sturm, Research Coordinator
kristin.sturm@cuanschutz.edu
University of Michigan
Principal Investigator: Dinesh Khanna, MD
Primary Contact People:
Rosemary Gedert, Research Coordinator
rgedert@med.umich.edu
James St. Clair, Research Coordinator
stclaija@med.umich.edu
Amisha Kaur, Research Coordinator
amikaur@med.umich.edu
Neda Kortam, Research Coordinator
nkortam@med.umich.edu
University of Minnesota
Principal Investigator: Jerry Molitor, MD
Primary Contact People:
Nicolas Rauwolf, Research Coordinator
rauwo004@umn.edu
Karen Omlung, Research Coordinator
seve0024@umn.edu
612.624.5617
University of Pennsylvania
Principal Investigator: Nora Sandorfi, MD
Primary Contact People:
Lauren Baker, Research Coordinator
Lauren.Baker@Pennmedicine.upenn.edu
Wren Catrillo, Research Coordinator
Wren.Catrillo@Pennmedicine.upenn.edu
Paris Mitchell, Research Coordinator
parisx@upenn.edu
Sally Thompson, Research Coordinator
sallyj@pennmedicine.upenn.edu
215.614.4430
University of Utah
Principal Investigator: Dorota Lebiedz-Odrobina, MD
Primary Contact People:
Inge Stijleman, Research Coordinator
inge.stijleman@hsc.utah.edu
Sally Bradstreet, Research Coordinator
Sally.Bradstreet@hsc.utah.edu
801.213.1368
Vanderbilt University Medical Center, Tennessee
Principal Investigator: Tracy Frech, MD
Primary Contact Person: Sarah Wood, Research Coordinator
sarah.wood@vumc.org
615.322.3635
WEBINAR SERIES
Learn About CONQUER
Watch an informative webinar explaining the importance of the CONQUER Registry and how it could lead to finding a cure for scleroderma.

How Does My Data Get Used, and Is It Protected?
- Your clinical data and blood samples will be de-identified so no one can get personal information about you.
- CONQUER data is protected in a secure data center at the University of Utah. This Data Coordinating Center (DCC), based at the University of Utah School of Medicine, provides a central repository for data generated by each of the CONQUER sites. The DCC also works with each of the CONQUER Principal Investigators and Research Coordinators to implement CONQUER-wide standards for data collection and analysis in order to ensure uniformity and quality of the data. The DCC implements regular monitoring activities and performs statistical analyses for many manuscripts published on behalf of CONQUER.
- CONQUER blood samples are stored separately at the University of Texas-Houston – the largest biorepository of scleroderma samples in the world.
What is the Benefit to Me?
- Your participation will help accelerate advances in clinical care
- This registry supports the development of new therapies
- Patients enrolled in CONQUER will experience heightened scrutiny and care in a formal setting
- In the future, CONQUER will include a clinical trials platform for rapid testing of new drugs and enrolled patients may be eligible to participate in these trials.
What Have We Learned From CONQUER So Far?
Researchers have already published papers based on analysis of CONQUER data.
Collaborative National Quality and Efficacy Registry (CONQUER) for Scleroderma: Outcomes from a Multicenter US-based Systemic Sclerosis Registry (2020)
By: Victoria K. Shanmugam, Tracy M. Frech, Virginia D. Steen, Laura K. Hummers, Ami A. Shah, Elana J. Bernstein, Dinesh Khanna, Jessica K. Gordon, Flavia V. Castelino, Lorinda Chung, Faye N. Hant, Emily Startup, John M. VanBuren, Luke B. Evnin & Shervin Assassi
Does hand involvement in Systemic Sclerosis limit PRO completion? (2021)
By: Tracy M. Frech, John M. VanBuren, Emily Startup, Shervin Assassi, Elana J. Bernstein, Flavia V. Castelino, Lorinda Chung, Chase Correia, Jessica K. Gordon, Faye N. Hant, Laura Hummers, Dinesh Khanna, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia Steen & Luke Evnin
Summary: The objective of this analysis is to examine whether the severity of systemic sclerosis (SSc)-hand involvement influences patient-reported outcome measure (PROM) completion rate. There were 339 CONQUER participants with total of 600 baseline and follow-up visits available for analysis. Fingertip resorption (acro-osteolysis) and calcium deposits (calcinosis) were associated with lower PROM completion, independent of the length of the questionnaires or the modality of administration (electronic or paper). Multiple strategies are needed to ensure optimal completion of PROM in longitudinal cohort studies.
Baseline Characteristics of Systemic Sclerosis Patients with Restrictive Lung Disease in a Multi-Center US Based Longitudinal Registry (2022)
By: Flavia V. Castelino, John M. VanBuren, Emily Startup, Shervin Assassi, Elana J. Bernstein, Lorinda Chung, Chase Correia, Luke B. Evnin, Tracy M. Frech, Jessica K. Gordon, Faye N. Hant, Laura K. Hummers, Dinesh Khanna, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia Steen
Summary: The researchers looked at the various characteristics associated with restrictive lung disease (RLD) in patients enrolled in CONQUER. There were no differences in age, gender or disease duration. Patients with restrictive lung disease had a mean disease duration of 2.6 years. Non-white race, higher physician global health assessment scores and dyspnea scores were independently associated with RLD. Interestingly, 45% of CONQUER participants already had RLD, highlighting the importance of screening for lung disease at initial diagnosis of systemic sclerosis.
Computed Tomography of the Chest to Screen for Interstitial Lung Disease in Patients with Systemic Sclerosis at Expert Scleroderma Centers in the U.S. (2022)
By: Elana J. Bernstein, Shervin Assassi, Flavia V. Castelino, Lorinda Chung, Chase Correia, Luke B. Evnin, Tracy M. Frech, Jessica K. Gordon, Brian A. Skaug, Faye N. Hant, Laura K. Hummers, Nora Sandorfi, Ami A. Shah, Victoria K. Shanmugam, Virginia D. Steen, Dinesh Khanna
Summary: Although a chest CT scan is the gold standard test for the diagnosis of interstitial lung disease, there is no consensus among rheumatologists regarding the use of chest CT to screen for interstitial lung disease in their patients with systemic sclerosis. The goal of this study was to describe the chest CT ordering practices at expert scleroderma centers in the United States and to determine which patient characteristics are associated with obtaining a chest CT. The researchers found that 80% of systemic sclerosis patients enrolled in CONQUER underwent a chest CT. Patients with a positive centromere antibody were less likely to have undergone a chest CT.
CONQUER Scleroderma: Association of Gastrointestinal Tract Symptoms in Early Disease with Resource Utilization (2023)
By: Sarah Luebker, Tracy M Frech, Shervin Assassi, Brian Skaug, Jessica K Gordon, Kimberly Lakin, Elana J Bernstein, Yiming Luo, Virginia D Steen, Ami A Shah, Laura K Hummers, Carrie Richardson, Duncan F Moore, Dinesh Khanna, Flavia V Castelino, Lorinda Chung, Puneet Kapoor, Faye N Hant, Victoria K Shanmugam, John M VanBuren, Jessica Alvey, Monica Harding, Ankoor Shah, Ashima Makol, Dorota Lebiedz-Odrobina, Julie K Thomas, Elizabeth R Volkmann, Jerry A Molitor, Nora Sandorfi
Summary: This report from the CONQUER cohort looked at how gastrointestinal tract (GIT) symptoms impacts health-related costs of care. Among the 211 CONQUER participants in this study, most (64%) had mild GIT symptoms, 26% had moderate symptoms, and 10% severe GIT symptoms at 12-months of follow-up. More upper endoscopy procedures and inpatient hospitalization occurred in the CONQUER participants with severe GIT symptoms. It is especially important to understand symptom activity prior to damage to the GIT and how diagnosis increases care costs.
The Collaborative National Quality and Efficacy Registry for Scleroderma: Association of Medication Use on Gastrointestinal Tract Symptoms in Early Disease and the Importance of Tobacco Cessation (2023)
By: S. Luebker, T.M. Frech, S. Assassi, J.K. Gordon, E.J. Bernstein, V.D. Steen,
A.A. Shah, L.K. Hummers, C. Richardson, D. Khanna, F.V. Castelino1, L. Chung, F.N. Hant, V.K. Shanmugam, J.M. VanBuren, J. Alvey, M. Harding, N. Sandorfi
Summary: This manuscript presents the gastrointestinal tract (GIT) outcomes in relation to other scleroderma manifestations and medication exposures. 415 enrolled CONQUER participants were included in this analysis. Most participants had stable mild GIT symptoms at baseline and were prescribed immunosuppression and anti-heartburn therapy. In most patients, anti-reflux medication and immunosuppression initiation preceded the baseline visit, whereas anti-fibrotic initiation occurred at or after the baseline visit. There was no clear association between immunosuppression or anti-fibrotic use and severity of GIT symptoms. However, active tobacco use was associated with worse GIT symptoms, highlighting the importance of smoking cessation counselling.
Still Want More Information About Conquer?
- Watch the CONQUER Webinar here
- Read more about it here
- Download our flier here
- Email info@srfcure.org for any questions you may have.
OUR RESEARCH
Dive Deeper into Our Research
Dig into the details of our scleroderma research program to understand more about the science behind what our elite researchers are doing to find better treatments and, ultimately, a cure.
Founder

Founding Sponsor

Supporting Partners



